Thanks to Brian Ford-Lloyd, Emeritus Professor of Plant Conservation Genetics at the School of Biosciences, University of Birmingham, for this contribution, hopefully the first of many.
Aside from beet cyst nematode, rhizomania is the most important disease of sugar beet worldwide, having plagued growers since the early 1950s. It can only be combated by growing resistant varieties, and there are two known major genes conferring resistance, one having been discovered by conventional means in the sugar beet crop, and the other in wild sea beet (Beta vulgaris ssp. maritima) populations in northern Europe. In a recent publication, Capistrano-Gossmann et al. (2017) have identified the actual wild beet gene involved (Rz2), using a complex but powerful molecular genetic process, a modified version of mapping-by-sequence together with the generation of a draft genome sequence and fine mapping. 1
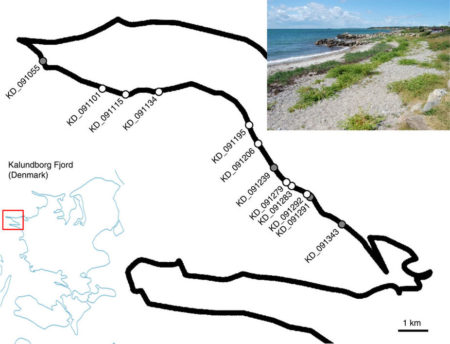
But let’s leave aside the detailed methodology, including what the gene actually encodes! As far as readers of this blog are concerned, what is the significance of this piece of research? It all started with the existing knowledge that a large population of sea beet in Denmark contained some plants that showed the resistance trait, and in my recollection this population had been studied for many years. But success depended upon sampling plants (189 of them) directly from the in situ population that covered a stretch of at least 10 kilometers of the Danish coast. The magic is that, compared to conventional synthetic breeding populations, this wild population possessed a distinct benefit — many generations of ‘random’ outcrossing resulted in low linkage disequilibrium and high population admixture. This was the key to successfully fine-mapping and genomically pinpointing the causal gene within the beet DNA sequence.
As the authors rightly point out, their research not only demonstrates the value of crop wild relatives, but it also highlights the need for ensuring that populations of these wild relatives are adequately conserved in their natural habitats and are subjected to appropriate and detailed evaluation for useful traits.
There are some important points that arise from this. Firstly, this particular use of a crop wild relative is not direct in the sense of transferring the gene by way of a plant breeding programme, but lies in the molecular isolation of the gene, that could then be subsequently transferred by whatever means, including genetic manipulation of one sort or another. Secondly, ‘evaluation’ of germplasm conserved in situ is something that has not received much attention, to my knowledge. And thirdly, preserving the population’s size and integrity would be important in maintaining its population genetic structure and ‘power’. Allowing it to go through a genetic bottleneck would diminish its value.
It is fortunate that wild sea beet is not categorised by IUCN as being under threat and large outbreeding populations do exist. The genetic potential of one or two other wild beets (Beta patula, for example), with smaller population sizes, is more in question.
- Gina G. Capistrano-Gossman et al. Crop wild relative populations of Beta vulgaris allow direct mapping of agronomically important genes. Nature Communications 8:15708. DOI:10.1038/ncomms15708
Brian Ford-Lloyd above: “Secondly, ‘evaluation’ of germplasm conserved in situ is something that has not received much attention, to my knowledge…”
Nigel Maxted, B.V. Ford-Lloyd, J.G. Hawkes (2013) p. 334. “We disagree with the scheme, advocated by Lenné and Wood (1991), that emphasizes in situ evaluation for resistance”
Nice to know people can change their minds. But I question whether three people based in Birmingham can question Lenné, one of the most experience plant pathologists with massive in situ evaluation experience. And what about the Am[m]iad project?
Very interesting insights from Brian Ford-Lloyd. But quite unnecessary – and slightly offensive – observations from Dave Wood.
Mike: The quote I gave from the 2013 book was not made by Maxted et al. but is in a chapter from their 2013 book by Dinoor. My mistake: I picked it up from a scrappy `Google Books’ file while searching from the Amiad project (which was an example of in situ evaluation). But Brian’s: “Secondly, ‘evaluation’ of germplasm conserved in situ is something that has not received much attention, to my knowledge” is wrong. If he means `in situ evaluation’ there are lots of examples of wild species – Lenné’s work all over the place, Burdon ditto – a life’s work, including on Glycine, and lots more. More to the point, farmers are conserving germplasm in situ everywhere and plant collectors are moving this ex situ for evaluation to the tune of millions of samples, exactly as done in the cited paper: “Crop wild relative populations of Beta vulgaris allow direct mapping of agronomically important genes” where almost 200 samples were collected for lab analysis – certainly not in situ evaluation. This is a modern version of Wickham sending 70,000 seeds of wild rubber from Brazil to Ceylon and Malaya in 1876 for evaluation and what a lot of us have being doing ever since. There would be far more value in conserving the wheat fields in Turkey – where to incredible disease resistances found by Harlan came from – than conserving – at huge cost –extensive populations of common CWRs. Sea Beet is not a good example of the need for conservation – far from it, it is all over the shores of southern Britain and elsewhere – I remember cooking and eating leaves from plants growing on shingle banks in Morecambe Bay in Lancashire – food for free.
I ask Jill for comments:
“1. The title is misleading. This is an example of sampling an in situ population of a wild Beta and screening plants ex situ for disease resistance. There are many examples of collection of wild plants and screening for disease resistance (see Jeremy Burdon’s extensive studies) – it is not uncommon.
2. It is possible to screen wild populations of economic plants in situ providing time for monitoring and funding are available. Dinoor (1970) efficiently identified adult plants of Avena sterilis resistant to Puccinia coronata in Israel in the 1970’s. Lenné (various references) identified wild Stylosanthes spp. accessions in Colombia and Brazil in the 1980’s. In contrast, the Ammiad Project conducted in Israel from 1984-1993 failed to identify resistance to leaf rust in wild emmer populations due to the very low incidence of disease. The key to finding useful genes for resistance in in situ wild populations is high disease pressure.
3. The occurrence of resistance genes in wild relatives of crops is evidence of powerful past selective forces (Harper, 1990) but how long past? It is very difficult to assess how far the concept of plant pathogen coevolution is justified in generating useful resistance since evolutionary changes may not be observed for 100 years or more (Frankel et al., 1995). Added to this there are interesting examples of disease resistance genes in wild populations that have never been exposed to the pathogen – allopatric resistance.
4. It is therefore questionable to place too much emphasis on in situ conservation of wild relatives of crops for the purposes of obtaining disease resistances in the future. Paradoxically, the chances of finding resistances are determined by increasing disease pressure which can harm such populations.”
Me again. One of the reasons for putting CGIAR Centres – and their genebanks – in regions of crop diversity was the high natural disease pressure, needed for resistance evaluation. The two pastures programmes (ILRI and CIAT) were screening wild species routinely – it certainly received massive attention.